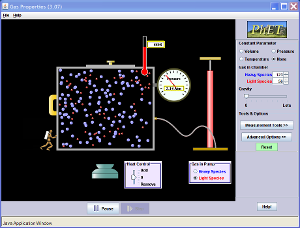
5.19 Ideal graph and conclusion09 November 201115:15
Sunday, 20 November 2011
5.19 Boyle's Law
5.19 Boyle's Law28 October 201111:11·5.19 use the relationship between the pressure and volume of a fixed mass of gas at constant temperature:p1V1 = p2V2
p1 = Pressure at the beginning [kPa, bar or atm]V1 = Volume at the beginning [m3 or cm3]p2 = Pressure at the end [kPa, bar or atm]V2 = Volume at the end [m3 or cm3]
(Note: can use any units for V and p as long as they are the same at the beginning and end)
5.19 Boyle's Law
5.19 Boyle's Law demos02 November 201120:01
Sunday, 13 November 2011
5.13
5.13 Starter·How can you fit a giraffe, 2 dogs and a swan into a standard laboratory beaker?!5.13 Starter 202 November 201118:17· Use particle theory to explain why the gas in the balloon contracts
Explanation
· The temperature of the gas inside the balloon decreases so the average speed of the particles decreases· Consequently the gas particles collide with the walls of the balloon with less force and less collisions per second· Because the walls of the container are flexible, the volume decreases5.13 Charles' law28 October 201111:10· 5.13 understand that there is an absolute zero of temperature which is –273oC
Open the Charles' law interactive experiment
· Adjust the temperature· What’s the relationship between temperature and volume?· Plot a graph of V against T· Take a screen shot of the graph
5.13 results and conclusion28 October 201111:10

Conclusion
· Volume is directly proportional to absolute (Kelvin) temperature· (gas is at constant pressure - flexible container)· V α T5.13 Plenary02 November 201119:13

Instructions
· Keep the Pressure constant (flexible container)· Pump some gas particles in· Predict what will happen when you heat the gas. Try it.· Predict what will happen when you cool the gas. Try it.
Question
· What would happen to the volume of the container if you could cool the gas to absolute zero, 0K?

Charles' law interactive experiment.swf Download this file
Thursday, 10 November 2011
5.11
5.11 Starter
>
· You're looking at smoke particles in air under a microscope
· They appear to be jiggling about
· Why?
· (Don't worry if you can't work this out straight away - Albert Einstein was the bloke who eventually explained what's happening here!) 5.11
· 5.11 understand the significance of Brownian motion
> Model 1
· What does the red puck represent?
· What do the metal balls represent?
[cid:image001.png@01CC9989.A14EFF60]
[cid:image002.png@01CC9989.A14EFF60]
> Model 3
· What do the "smoke" particles look like?
· Why are they moving?
· What do the "air" particles look like? 5.11 explained
Model 1
· What does the red puck represent?
o The large, visible smoke particle
· What do the metal balls represent?
o The small, not visible air particles Model 2
· What do the small red particles represent?
o The small, not visible air particles
· What does the large blue particle represent?
o The large, visible smoke particle
· What does the view on the left of the screen represent?
o The view through the microscope lense
· Why can‘t you see the red particles in this view?
o They are too small to see Model 3
· What do the "smoke" particles look like?
o They are the 5 large, sand coloured particles
· Why are they moving?
o Small, fast moving air particles are colliding with the smoke particles and making them move
· What do the "air" particles look like?
o They are the numerous, small, white particles 5.11 Questions
1. Draw the path of a smoke particle in air (3 marks)
2. Explain what is meant by Brownian Motion of smoke particles in air and how it provides evidence for air particles (4 marks)
3. What change would you expect to see in the movement of the smoke particles if the air was cooled down? Why? (2 marks) 
>
· You're looking at smoke particles in air under a microscope
· They appear to be jiggling about
· Why?
· (Don't worry if you can't work this out straight away - Albert Einstein was the bloke who eventually explained what's happening here!) 5.11
· 5.11 understand the significance of Brownian motion
> Model 1
· What does the red puck represent?
· What do the metal balls represent?
[cid:image001.png@01CC9989.A14EFF60]
[cid:image002.png@01CC9989.A14EFF60]
> Model 3
· What do the "smoke" particles look like?
· Why are they moving?
· What do the "air" particles look like? 5.11 explained
Model 1
· What does the red puck represent?
o The large, visible smoke particle
· What do the metal balls represent?
o The small, not visible air particles Model 2
· What do the small red particles represent?
o The small, not visible air particles
· What does the large blue particle represent?
o The large, visible smoke particle
· What does the view on the left of the screen represent?
o The view through the microscope lense
· Why can‘t you see the red particles in this view?
o They are too small to see Model 3
· What do the "smoke" particles look like?
o They are the 5 large, sand coloured particles
· Why are they moving?
o Small, fast moving air particles are colliding with the smoke particles and making them move
· What do the "air" particles look like?
o They are the numerous, small, white particles 5.11 Questions
1. Draw the path of a smoke particle in air (3 marks)
2. Explain what is meant by Brownian Motion of smoke particles in air and how it provides evidence for air particles (4 marks)
3. What change would you expect to see in the movement of the smoke particles if the air was cooled down? Why? (2 marks)

brownian_motion.swf Download this file
Monday, 7 November 2011
5.12 + 5.15
5.12+5.15 Starter 02 November 2011 16:15 > Questions
· Why does the needle on the meter move when gas particles are introduced into the box?
· What does the meter measure? Answers
· The gas particles collide with all of the walls of the container. The wall on the right moves outwards and moves the needle.
· Pressure. The gas particles colliding with the walls makes a force on the walls. The walls have a surface area so the quantity measured is pressure, p=F/A. 5.12+5.15 Questions 02 November 2011 15:55
· 5.12 recall that molecules in a gas have a random motion and that they exert a force and hence a pressure on the walls of the container
· 5.15 understand that an increase in temperature results in an increase in the speed of gas molecules
[cid:image001.png@01CC9986.E18EA0B0] Try the animation http://www.lon-capa.org/~mmp/kap10/cd283.htm
1. How do the particles create a pressure?
2. If you increase the temperature, how does the movement of the particles change?
3. If you increase the temperature, how does the number of collisions per second change?
4. If you increase the temperature, what does this do to the pressure? 5.12+5.15 Plenary 02 November 2011 15:55 > 
· Why does the needle on the meter move when gas particles are introduced into the box?
· What does the meter measure? Answers
· The gas particles collide with all of the walls of the container. The wall on the right moves outwards and moves the needle.
· Pressure. The gas particles colliding with the walls makes a force on the walls. The walls have a surface area so the quantity measured is pressure, p=F/A. 5.12+5.15 Questions 02 November 2011 15:55
· 5.12 recall that molecules in a gas have a random motion and that they exert a force and hence a pressure on the walls of the container
· 5.15 understand that an increase in temperature results in an increase in the speed of gas molecules
[cid:image001.png@01CC9986.E18EA0B0] Try the animation http://www.lon-capa.org/~mmp/kap10/cd283.htm
1. How do the particles create a pressure?
2. If you increase the temperature, how does the movement of the particles change?
3. If you increase the temperature, how does the number of collisions per second change?
4. If you increase the temperature, what does this do to the pressure? 5.12+5.15 Plenary 02 November 2011 15:55 >

Ideal gases - summary of terms.pptx Download this file
Saturday, 5 November 2011
Friday, 4 November 2011
5.9 and 5.10
5.9 and 5.10 starter Tell the person next to you…
· How do particles in move in a solid, a liquid and a gas?
· Describe…
o speed of particles
o relative position of particles (fixed or not)
o pattern of particles (regularly arranged or not)
o size of the particles
o space between the particles
o strength of bonds between the particles 
· How do particles in move in a solid, a liquid and a gas?
· Describe…
o speed of particles
o relative position of particles (fixed or not)
o pattern of particles (regularly arranged or not)
o size of the particles
o space between the particles
o strength of bonds between the particles

s,l,g animation.swf Download this file
Wednesday, 2 November 2011
5.7 and 5.8
5.7 and 5.8
· 5.7 understand that a substance can change state from solid to liquid by the process of melting
· 5.8 understand that a substance can change state from liquid to gas by the process of evaporation or boiling · Use following pages from Collins as a resource to help you
[cid:image019.jpg@01CC9576.07199B60]
[cid:image020.jpg@01CC9576.07199B60]
[cid:image021.jpg@01CC9576.07199B60]
[cid:image022.jpg@01CC9576.07199B60]
[cid:image023.jpg@01CC9576.07199B60] 5.7 and 5.8 Experiment - Cooling Curve of Stearic Acid using data logger
[cid:image024.jpg@01CC9576.07199B60] 


· 5.7 understand that a substance can change state from solid to liquid by the process of melting
· 5.8 understand that a substance can change state from liquid to gas by the process of evaporation or boiling · Use following pages from Collins as a resource to help you
[cid:image019.jpg@01CC9576.07199B60]
[cid:image020.jpg@01CC9576.07199B60]
[cid:image021.jpg@01CC9576.07199B60]
[cid:image022.jpg@01CC9576.07199B60]
[cid:image023.jpg@01CC9576.07199B60] 5.7 and 5.8 Experiment - Cooling Curve of Stearic Acid using data logger
[cid:image024.jpg@01CC9576.07199B60]

states of matter drag and drop plenary.swf Download this file

Fill the trucks - Properties of s,l,g.swf Download this file

Subscribe to:
Comments (Atom)